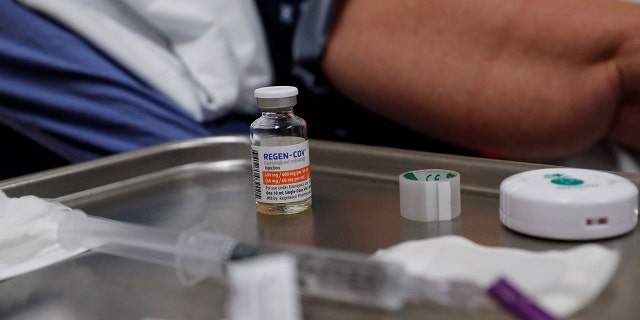
Guidance issued by the Biden administration states certain individuals may be considered “high risk” and more quickly qualify for monoclonal antibodies and oral antivirals used to treat COVID-19 based on their “race or ethnicity.”
In a fact sheet issued for healthcare providers by the Food and Drug Administration, the federal agency approved emergency use authorizations of sotrovimab – a monoclonal antibody proven to be effective against the Omicron variant – only to patients considered “high risk.”